CAR T-cell therapy is a new way to fight cancer, giving hope to those with blood cancers. It uses the body’s immune system to find and kill cancer cells. More and more cancers can be treated with this method, thanks to FDA-approved therapies.
This therapy works well against leukemia, lymphoma, and multiple myeloma. It changes T-cells to attack cancer cells, leading to high remission rates. As more trials happen and new therapies get approved, more cancers can be treated. This brings hope to patients and their families.
Understanding CAR T-Cell Therapy
CAR T-cell therapy is a new way to fight cancer. It uses your own immune system to attack cancer cells. This method is different from old treatments like chemotherapy, which harm both good and bad cells.
The car t-cell therapy process starts by taking your T-cells. These are special white blood cells that help fight off infections. They are then changed in a lab to find and attack cancer cells.
After the T-cells are changed, they are put back into your body. They multiply and search for cancer cells everywhere. When they find one, they attach and start an immune response that kills the cancer cell.
“CAR T-cell therapy represents a significant advancement in personalized cancer treatment, giving hope to those who have tried everything else.” – Dr. Sarah Johnson, oncologist
One big plus of CAR T-cell therapy is it can lead to long-term remission. Many patients in trials have seen their cancer completely go away. This is because the therapy creates a lasting army of T-cells to fight cancer.
| Feature | CAR T-Cell Therapy | Traditional Cancer Treatments |
|---|---|---|
| Specificity | Targets cancer cells only | Harms both good and bad cells |
| Durability | Can lead to long-term remission | Often needs ongoing treatment |
| Personalization | Uses your own immune cells | One-size-fits-all approach |
Even though CAR T-cell therapy is a success for some blood cancers, scientists are working to use it for other cancers too. As we learn more about how car t-cell therapy works, it looks very promising for the future of cancer treatment.
How CAR T-Cell Therapy Works
CAR T-cell therapy is a new way to fight cancer. It uses the body’s immune system to attack cancer cells. First, the patient’s T-cells are changed to find and destroy cancer cells.
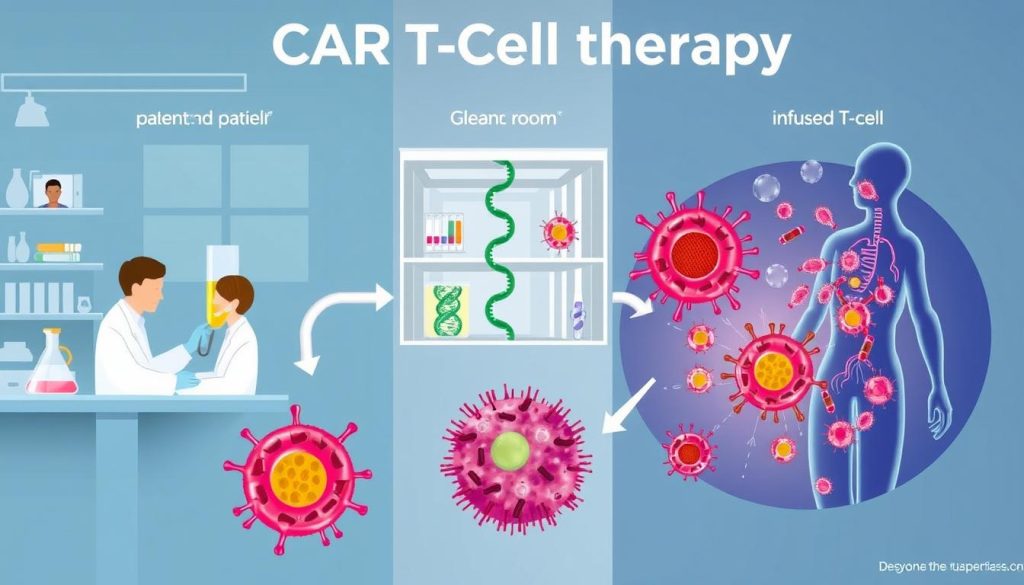
Genetically Modifying T-Cells
The first step is to take T-cells from the patient. This is done through a process called leukapheresis. Then, these cells are sent to a lab to be changed.
In the lab, the T-cells are made to have a special receptor. This receptor helps them find and kill cancer cells. A virus is used to add this receptor to the T-cells’ DNA.
Targeting Cancer Cells
After the T-cells are changed, they are grown in the lab. Millions of these cells are made to fight cancer. Then, they are given back to the patient.
These cells look for cancer cells to attack. When they find one, they bind to it and start killing it. This process helps get rid of cancer cells without harming healthy ones.
| CAR T-Cell Target | Cancer Type |
|---|---|
| CD19 | B-cell malignancies (leukemia, lymphoma) |
| BCMA | Multiple myeloma |
The CAR T-cells kill cancer cells and call for more immune cells to help. This targeted approach is more precise and less harmful than traditional treatments.
FDA Approved CAR T-Cell Therapies
CAR T-cell therapy has changed how we treat some blood cancers. By 2023, the FDA approved five CAR T-cell therapies for different blood cancers. These treatments give hope to those who have tried everything else.
Kymriah (tisagenlecleucel)
Kymriah, made by Novartis, was the first CAR T-cell therapy approved. It was approved in August 2017 for kids and young adults with a certain type of leukemia. In May 2018, it also got approval for adults with a certain type of lymphoma.
Yescarta (axicabtagene ciloleucel)
Yescarta, from Kite Pharma, a Gilead company, was approved in October 2017. It helps adults with a certain type of lymphoma that didn’t respond to other treatments.
Tecartus (brexucabtagene autoleucel)
Tecartus, also from Kite Pharma, was approved in July 2020. It’s for adults with a certain type of lymphoma. In October 2021, it also got approval for another type of leukemia.
Breyanzi (lisocabtagene maraleucel)
Breyanzi, from Bristol Myers Squibb, was approved in February 2021. It’s for adults with a certain type of lymphoma. It helps those who didn’t get better with other treatments.
Abecma (idecabtagene vicleucel)
Abecma, from Bristol Myers Squibb and bluebird bio, was approved in March 2021. It’s the first CAR T-cell therapy for multiple myeloma. It helps adults who have tried many treatments before.
These CAR T-cell therapies have shown great results in treating blood cancers. They offer hope to those who have tried everything else. As research goes on, we can expect these treatments to help even more people.
CAR T-Cell Therapy for Leukemia
CAR T-cell therapy is a new hope for those fighting leukemia. It uses the body’s immune system to fight cancer cells. This method targets and kills leukemia cells.
CAR T-cell therapy for leukemia is most promising for acute lymphoblastic leukemia (ALL). Studies show it can lead to complete remission for many patients. It works by changing T-cells to find and attack ALL cells.

Chronic lymphocytic leukemia (CLL) also sees benefits from CAR T-cell therapy. While it’s not as effective as in ALL, it’s a good option for those who’ve tried other treatments.
The FDA has approved two CAR T-cell products for leukemia:
- Kymriah (tisagenlecleucel) for relapsed or refractory ALL in kids and young adults
- Tecartus (brexucabtagene autoleucel) for relapsed or refractory CLL in adults
Scientists are working to make CAR T-cell therapy even better. They aim to save more lives from this deadly disease.
CAR T-Cell Therapy for Lymphoma
CAR T-cell therapy is a new hope for lymphoma patients. It uses the immune system to fight cancer cells. This method has shown great results in treating lymphoma.
Diffuse Large B-Cell Lymphoma (DLBCL)
DLBCL is the most common non-Hodgkin lymphoma. CAR T-cell therapy has shown great success in treating it. Patients with refractory or relapsed DLBCL have seen long-term remissions.
Primary Mediastinal B-Cell Lymphoma (PMBCL)
PMBCL is a rare and aggressive lymphoma. CAR T-cell therapy has been successful in treating it. It offers hope to those who didn’t respond to traditional treatments.
Follicular Lymphoma
Follicular lymphoma is slow-growing but can be hard to treat. CAR T-cell therapy has shown promising results. It offers an alternative to traditional treatments.
Mantle Cell Lymphoma
Mantle cell lymphoma is aggressive and has high relapse rates. Early studies suggest CAR T-cell therapy can induce remissions. It’s a much-needed option for this disease.
As research continues, CAR T-cell therapy is changing lymphoma treatment. It uses the immune system to deliver personalized treatment. This approach offers hope for lymphoma patients.
CAR T-Cell Therapy for Multiple Myeloma
Multiple myeloma, a blood cancer, has a new hope in car t-cell therapy for multiple myeloma. This treatment uses the patient’s immune system to fight cancer. It’s a new option for those who’ve tried everything else.
Treating multiple myeloma with car t-cell therapy starts with taking the patient’s T-cells. These cells are then changed to target myeloma cells. After, they’re put back into the patient to fight cancer.
The FDA approved Abecma (idecabtagene vicleucel) for car t-cell therapy for multiple myeloma. It’s for patients who’ve tried four treatments before. In tests, 72% of patients showed a good response, and 28% got a complete response.
“CAR T-cell therapy has given me a second chance at life. After exhausting all other treatment options, this innovative approach has brought me into remission and restored my hope for the future.” – Maria, a multiple myeloma survivor treated with CAR T-cell therapy.
While treating multiple myeloma with car t-cell therapy works well, it can have side effects. Patients might face cytokine release syndrome (CRS) or neurological issues. These need careful watching and management by a skilled team.
Scientists are working hard to make car t-cell therapy for multiple myeloma even better. They aim to improve its safety and effectiveness, giving patients more hope against this tough disease.
What Cancers Can Be Treated with CAR T-Cell Therapy
CAR T-cell therapy is a new way to fight cancer. It uses the body’s immune system to attack cancer cells. This method is approved for some cancers and is being tested for more.
Current FDA Approved Indications
Right now, CAR T-cell therapy is approved for certain blood cancers. These include:
- Acute Lymphoblastic Leukemia (ALL)
- Diffuse Large B-Cell Lymphoma (DLBCL)
- Primary Mediastinal B-Cell Lymphoma (PMBCL)
- Follicular Lymphoma
- Mantle Cell Lymphoma
- Multiple Myeloma
Studies have shown it works well for these cancers. It can lead to long-term remissions for patients who have tried other treatments.
Potential Future Applications
Scientists are looking into using CAR T-cell therapy for more cancers. They are testing it in solid tumors like:
| Cancer Type | Clinical Trial Status |
|---|---|
| Glioblastoma | Phase I/II |
| Pancreatic Cancer | Phase I |
| Breast Cancer | Phase I/II |
| Prostate Cancer | Phase I |
These early trials are promising. They could make CAR T-cell therapy available for more cancer types in the future.
CAR T-Cell Therapy Success Rates
CAR T-cell therapy has shown great success in treating blood cancers. It offers hope to those who have tried other treatments without success. Clinical trials and real-world data show high response rates and long-term remissions in patients with leukemia, lymphoma, and multiple myeloma.
The success of CAR T-cell therapy depends on the cancer type and the patient’s condition. Yet, many patients have seen significant improvements after this treatment.
In B-cell acute lymphoblastic leukemia (B-ALL) trials, CAR T-cell therapy has shown remission rates of 70% to 90%. For diffuse large B-cell lymphoma (DLBCL), response rates are around 50% to 60%. Many patients achieve complete remissions.
| Cancer Type | Overall Response Rate | Complete Remission Rate |
|---|---|---|
| B-cell Acute Lymphoblastic Leukemia (B-ALL) | 70-90% | 60-80% |
| Diffuse Large B-cell Lymphoma (DLBCL) | 50-60% | 30-40% |
| Multiple Myeloma | 60-80% | 40-50% |
While these success rates are promising, CAR T-cell therapy is not a cure for everyone. Some patients may not respond or experience a relapse. Research continues to improve its effectiveness and expand its use to more cancers.
The success of CAR T-cell therapy has been a game-changer for many patients who had run out of options. It’s giving them a second chance at life.
As more trials are done and long-term data is collected, we’ll understand CAR T-cell therapy’s lasting impact on cancer treatment and patient outcomes better.
CAR T-Cell Therapy Side Effects
CAR T-cell therapy is a promising treatment for some blood cancers. But, it can cause serious side effects. These happen because the immune system reacts strongly to the modified T-cells. The most common side effects include:
Cytokine Release Syndrome (CRS)
Cytokine release syndrome is a major side effect of CAR T-cell therapy. It happens when the immune system releases many cytokines. This can cause fever, chills, low blood pressure, and trouble breathing.
CRS can be mild or very serious. Managing it often requires careful monitoring and supportive care.
Neurological Toxicities
Neurological side effects can also occur. These might include confusion, tremors, seizures, or changes in consciousness. These effects are thought to be due to the immune system’s reaction and the presence of CAR T-cells in the brain.
Most of the time, these side effects can be managed. But in rare cases, they can be severe or last a long time.
B-Cell Aplasia
B-cell aplasia is a side effect of CAR T-cell therapies targeting CD19. This protein is on both cancerous and healthy B-cells. The therapy can deplete healthy B-cells, increasing the risk of infections.
This side effect is managed with immunoglobulin replacement therapy. This helps until the B-cells recover.
| Side Effect | Symptoms | Management |
|---|---|---|
| Cytokine Release Syndrome | Fever, chills, low blood pressure, difficulty breathing | Close monitoring, supportive care |
| Neurological Toxicities | Confusion, tremors, seizures, altered consciousness | Appropriate management, usually resolves |
| B-Cell Aplasia | Increased risk of infections | Immunoglobulin replacement therapy |
Even with these side effects, CAR T-cell therapy can be beneficial. It’s often used for patients with advanced blood cancers who have tried other treatments. It’s important to closely monitor and manage side effects to ensure the therapy’s success.
CAR T-Cell Therapy Process
CAR T-cell therapy is a detailed process. It involves several steps to modify a patient’s immune cells. This makes them target and destroy cancer cells. Here’s a look at the car t-cell therapy process:
T-Cell Collection
The first step is T-cell collection. Blood is taken from the patient. Then, T cells are separated from other blood components using a special machine.
Genetic Modification
After collecting T cells, they go to a lab for genetic modification. Scientists use a viral vector to add the CAR gene. This lets the T cells produce CARs on their surface.
Cell Expansion
Next, the CAR T cells multiply in the lab. This is called cell expansion. It ensures there are enough cells to fight the cancer.
Lymphodepletion
While preparing the CAR T cells, the patient gets chemotherapy. This treatment reduces immune cells. It makes room for the CAR T cells to work better.
CAR T-Cell Infusion
Lastly, the patient gets an infusion of CAR T cells. They are given through an IV, like a blood transfusion. The CAR T cells then find and destroy cancer cells.
| Step | Description |
|---|---|
| T-Cell Collection | Blood is drawn, and T cells are separated using a cell separator |
| Genetic Modification | T cells are genetically modified to express chimeric antigen receptors (CARs) |
| Cell Expansion | CAR T cells are stimulated to multiply in the laboratory |
| Lymphodepletion | Patient undergoes chemotherapy to reduce immune cells and make room for CAR T cells |
| CAR T-Cell Infusion | Patient receives an infusion of their genetically modified CAR T cells |
The entire CAR T-cell therapy process, from T-cell collection to infusion, typically takes several weeks to complete.
CAR T-Cell Therapy Cost
The cost of CAR T-cell therapy is a big worry for patients and their families. This treatment is very personalized and new, which makes it expensive. It’s important to know the costs and insurance coverage for those thinking about it.
Treatment Expenses
The cost of CAR T-cell therapy can change based on a few things. These include the treatment product, the patient’s health, and where the treatment is given. On average, it costs between $373,000 and $475,000 per treatment. This includes:
- T-cell collection and genetic modification
- Cell expansion and preparation
- Lymphodepletion chemotherapy
- CAR T-cell infusion
- Hospital stay and supportive care
Other costs might include tests before treatment, checks after treatment, and managing side effects.
Insurance Coverage
Insurance for CAR T-cell therapy can vary a lot. Many private insurance plans and Medicare now cover it for certain uses. But, coverage might need approval and certain criteria.
Patients should talk to their healthcare team and insurance about what’s covered. Some places also offer help with costs or payment plans.
| CAR T-Cell Product | Approximate Cost | Medicare Coverage |
|---|---|---|
| Kymriah | $475,000 | Yes |
| Yescarta | $373,000 | Yes |
| Tecartus | $373,000 | Yes |
| Breyanzi | $410,300 | Yes |
| Abecma | $419,500 | Yes |
Even though CAR T-cell therapy is expensive, it’s worth considering. For many with advanced blood cancers, it offers a chance at long-term remission or even a cure when other treatments fail.
Eligibility for CAR T-Cell Therapy
CAR T-cell therapy is a breakthrough for some blood cancers. But, not everyone can get this treatment. Eligibility for CAR T-cell therapy depends on several factors. These include the cancer type and stage, age, and past treatments.
Patients with specific blood cancers might be eligible. These include leukemia, lymphoma, or multiple myeloma. They must not have responded well to standard treatments or have relapsed. The FDA has approved CAR T-cell therapies for certain cases.
- Relapsed or refractory diffuse large B-cell lymphoma (DLBCL)
- Relapsed or refractory follicular lymphoma
- Relapsed or refractory mantle cell lymphoma
- Relapsed or refractory multiple myeloma
- Relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in patients up to 25 years old

Age is also a factor. Some treatments are for both kids and adults, while others have age limits. For example, Kymriah is for patients up to 25 with B-cell ALL. Yescarta and Tecartus are for adults with certain lymphomas.
“The decision to pursue CAR T-cell therapy should be made in close consultation with a healthcare team experienced in this specialized treatment.”
Doctors will check a patient’s health before suggesting CAR T-cell therapy. They look at organ function and how well the patient can handle treatment side effects. Patients also need enough healthy T-cells for the treatment to work.
CAR T-Cell Therapy Clinical Trials
CAR T-cell therapy is showing great promise in fighting cancer. Researchers are working hard in many clinical trials. They aim to make this treatment better, safer, and more available to those who need it.
Ongoing Research
Researchers are focusing on several important areas in CAR T-cell therapy trials. They are working to:
- Make CAR T-cells target different cancer antigens
- Improve how long CAR T-cells last and how well they work
- Lower the chance of side effects like cytokine release syndrome
- Look into using “off-the-shelf” CAR T-cells
- Test CAR T-cells with other treatments
By tackling these challenges, they hope to make CAR T-cell therapy better for more cancers.
Expanding Treatment Options
Right now, CAR T-cell therapy is approved for some cancers like leukemia, lymphoma, and multiple myeloma. But, trials are looking into using it for other cancers too. This includes:
| Cancer Type | Clinical Trial Phase |
|---|---|
| Solid tumors (e.g., breast, lung, pancreatic) | Phase 1/2 |
| Brain tumors (e.g., glioblastoma) | Phase 1 |
| Ovarian cancer | Phase 1/2 |
| Prostate cancer | Phase 1 |
As these car t-cell therapy clinical trials move forward, they could open up this treatment to more people. This could lead to better survival rates and a better quality of life for many.
Advancements in CAR T-Cell Therapy
The field of CAR T-cell therapy has seen big steps forward. This gives hope to those fighting cancer. Researchers and companies are working hard to make this treatment better, safer, and more accessible.
Improving how CAR T-cells are made is a key area of focus. Scientists aim to make this process faster and cheaper. This could help more people get the treatment they need.
Creating off-the-shelf CAR T-cell therapies is another big step. Right now, treatments use a patient’s own T-cells. But, researchers are making “universal” CAR T-cells from healthy donors. This could make treatment faster, cheaper, and more available.
The future of CAR T-cell therapy is bright. It might be used with other treatments like immune checkpoint inhibitors and targeted therapies. This could make cancer treatment even more effective and improve patient results.
“The advancements in CAR T-cell therapy are truly remarkable. We are witnessing a new era in cancer treatment, where we can harness the power of the immune system to fight this devastating disease.” – Dr. Emily Thompson, a leading researcher in the field of CAR T-cell therapy.
As research keeps moving forward, CAR T-cell therapy’s future looks bright. With more clinical trials and teamwork, we’ll see even more progress. These advances will help treat more cancers and improve patient outcomes.
Comparing CAR T-Cell Therapy to Other Treatments
When looking at cancer treatment options, it’s key to know how CAR T-cell therapy stacks up against others. Chemotherapy, stem cell transplant, and targeted therapies have long been the go-to. But CAR T-cell therapy brings a new, hopeful approach for some cancers.
Chemotherapy
Chemotherapy uses strong drugs to kill fast-growing cells, like cancer. But it can harm healthy cells too, causing hair loss, nausea, and tiredness. CAR T-cell therapy targets cancer cells more precisely, possibly hurting fewer healthy tissues. It might be a better choice for some patients than traditional chemotherapy.
Stem Cell Transplant
Stem cell transplants replace bad bone marrow with healthy cells. They work well for some blood cancers but come with risks like infections and graft-versus-host disease. CAR T-cell therapy uses the patient’s own immune cells to fight cancer, possibly avoiding these transplant issues.
Targeted Therapies
Targeted therapies attack cancer cells based on their unique traits, like genetic mutations. They’re effective for some cancers but not all. CAR T-cell therapy uses the immune system to find and kill cancer cells. As research grows, combining CAR T-cell therapy with targeted therapies could lead to even better treatments.
FAQ
Q: What types of cancer can be treated with CAR T-cell therapy?
A: CAR T-cell therapy is approved for some cancers. These include leukemia, lymphoma, and multiple myeloma. It’s used for acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and more.
Q: How effective is CAR T-cell therapy in treating cancer?
A: CAR T-cell therapy has shown great success in treating blood cancers. It works well for patients who have tried other treatments without success. But, its success can vary based on the cancer type and the patient.
Q: What are the possible side effects of CAR T-cell therapy?
A: Side effects include cytokine release syndrome (CRS), which can cause fever and breathing issues. Neurological toxicities may lead to confusion and seizures. B-cell aplasia is a long-term risk that can increase infection chances.
Q: How much does CAR T-cell therapy cost?
A: CAR T-cell therapy is very expensive, often over 0,000. But, many insurance plans, including Medicare, cover it for eligible patients. It’s important to talk about costs and insurance with your healthcare team.
Q: How long does the CAR T-cell therapy process take?
A: The process takes several weeks. It includes collecting T-cells, modifying them, expanding the cells, and a final infusion. Patients need to stay close to the treatment center for monitoring.
Q: Who is eligible for CAR T-cell therapy?
A: Eligibility depends on cancer type, stage, age, and previous treatments. It’s for patients with certain blood cancers who haven’t responded to other treatments.
Q: Are there ongoing clinical trials for CAR T-cell therapy?
A: Yes, many trials are exploring CAR T-cell therapy for different cancers. They aim to find new uses and improve outcomes. Trials are looking at solid tumors and combining CAR T-cell therapy with other treatments.
Q: How does CAR T-cell therapy compare to other cancer treatments?
A: CAR T-cell therapy is a targeted treatment with great results for some blood cancers. It offers long-term remission with a single infusion. But, it comes with unique risks and side effects that need careful management.