In the fight against cancer, CAR T cell therapy is a new hope for patients with certain blood cancers. It uses the body’s immune system to target and destroy cancer cells with great precision. CAR T cell therapy, short for chimeric antigen receptor T cell immunotherapy, is a personalized treatment. It genetically modifies a patient’s T cells to attack specific cancer cells. Knowing how it works helps patients and their families make informed choices about treatment.
This guide will cover the basics of CAR T cell treatment, its development, and the cancers it can treat. It will also discuss the benefits and risks of this therapy. As research advances, CAR T cell therapy could change cancer care and improve patient outcomes worldwide.
Understanding the Basics of CAR T Cell Therapy
CAR T cell therapy is a new way to treat blood cancers like leukemia and lymphoma. It uses the patient’s immune system to fight cancer. Knowing how car t cells explained work helps patients and families understand this therapy.
How CAR T Cell Therapy Works
The therapy starts by taking T cells from the patient’s blood. These cells are then changed in a lab to find and kill cancer cells. This is done by adding chimeric antigen receptors (CARs) to their surface.
After the T cells are made, they are grown in the lab. The patient gets chemotherapy to make room for these new cells. Then, the CAR T cells are given back to the patient to fight cancer.
The Role of T Cells in the Immune System
T cells are key in fighting off infections and cancer. But, cancer cells can hide from T cells. This lets cancer grow and spread.
“The beauty of CAR T cell therapy is that it enhances the natural ability of T cells to fight cancer, giving patients a powerful new weapon in their battle against the disease.” – Dr. Sarah Johnson, oncologist
By changing T cells to have CARs, car t therapy for cancer helps them find and kill cancer cells better. This method has shown great success in treating blood cancers. It offers hope to those who have tried other treatments.
Even though CAR T cell therapy is a form of adoptive cell transfer therapy, it’s special because of its genetic engineering. This makes it a strong tool in fighting cancer, marking a new area in cancer treatment.
The Development of CAR T Cell Therapy
The field of engineered t cell therapy has seen huge progress in recent decades. It has changed the way we fight cancer with personalized treatments. This journey has been filled with scientific breakthroughs, innovation, and hard work by researchers and doctors around the world.

History and Evolution of CAR T Cell Research
The story of CAR T cell therapy started in the 1980s. Back then, scientists were looking into how to modify T cells to attack specific cancer cells. They were studying how T cells work and their role in fighting cancer.
In the 1990s, the idea of chimeric antigen receptors (CARs) came up. This idea was key to creating CAR T cell therapy. CARs combine parts of antibodies and T cell receptors. This lets T cells find and destroy cancer cells more effectively.
“The advent of CAR T cell therapy has opened up new possibilities in the fight against cancer, giving hope to those who have tried everything else.” – Dr. Michel Sadelain, Memorial Sloan Kettering Cancer Center
Key Milestones in CAR T Cell Therapy Development
There have been many important moments in the development of CAR T cell therapy:
- In the early 2000s, the first CAR T cell therapy trials started. They focused on CD19, a protein found on B cell cancers.
- In 2010, a groundbreaking case showed CAR T cells could cure advanced CLL. This was a big step towards long-term remissions.
- In 2017, the FDA approved Kymriah and Yescarta for treating certain cancers. This was a major win for personalized cancer treatment.
After that, CAR T cell therapy has kept growing. Scientists are working hard to use it for more cancers. They also want to make it safer and more effective. New ways to improve CAR T cells are being explored.
Types of Blood Cancers Treated with CAR T Cell Therapy
CAR T cell therapy has shown great success in treating blood cancers like leukemia and lymphoma. These cancers affect the blood, bone marrow, and lymphatic system. They can be hard to treat with traditional methods like chemotherapy and radiation.
One of the most promising uses of CAR T cell therapy is in treating acute lymphoblastic leukemia (ALL). This cancer mainly affects children and young adults. In clinical trials, CAR T cell therapy for leukemia has achieved remission rates of up to 90% in patients who had tried all other treatments.
Another blood cancer that has shown promising results with CAR T cell therapy is diffuse large B-cell lymphoma (DLBCL). This is an aggressive form of non-Hodgkin’s lymphoma. CAR T cell therapy for lymphoma has shown the ability to induce long-term remissions in patients with refractory or relapsed DLBCL.
The following table highlights some of the key clinical trials involving CAR T cell therapy for blood cancers:
| Clinical Trial | Cancer Type | Remission Rate |
|---|---|---|
| ELIANA | Pediatric ALL | 81% |
| ZUMA-1 | Adult DLBCL | 54% |
| JULIET | Adult DLBCL | 40% |
The success of CAR T cell therapy in treating blood cancers can be attributed to several factors. Blood cancers originate from the immune system itself, making them more susceptible to immunotherapy approaches. The specific antigens targeted by CAR T cells, such as CD19, are mainly expressed on cancerous B cells. This minimizes off-target effects on healthy tissues.
“The remarkable responses observed in patients with advanced blood cancers treated with CAR T cell therapy have given hope to many who had run out of options.” – Dr. James Carlton, Oncologist
As research continues, scientists are exploring ways to expand the use of CAR T cell therapy to other types of blood cancers and even solid tumors. The success seen in treating leukemia and lymphoma with CAR T cells has paved the way for a new era in cancer treatment. It offers personalized and targeted approaches to fighting this devastating disease.
The CAR T Cell Therapy Process
CAR T cell therapy is a cutting-edge treatment that uses a patient’s immune system to fight cancer. It starts with collecting T cells and ends with car t cell infusion. This is when the modified cells are put back into the patient’s body.
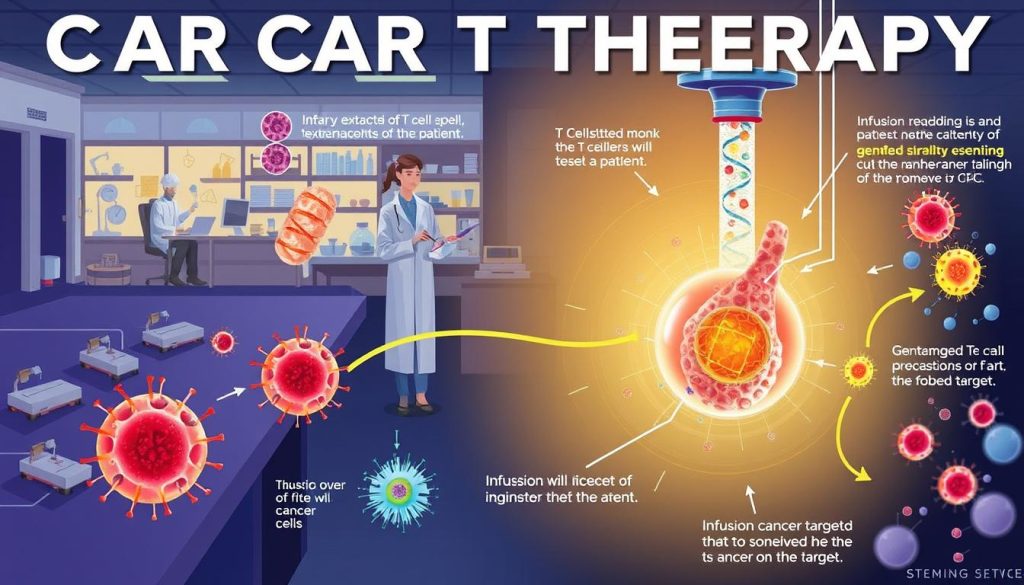
Step 1: T Cell Collection
The first step is collecting the patient’s T cells through leukapheresis. This process draws blood, separates T cells, and returns the rest to the patient.
Step 2: Genetic Modification of T Cells
After collecting T cells, they go to a lab for genetic modification. Scientists use a viral vector to introduce the CAR into the T cells. This makes them recognize and target cancer cells.
Step 3: Multiplication of CAR T Cells
The modified T cells are then multiplied in the lab. This creates millions of CAR T cells. This is to ensure there are enough to fight cancer when infused back into the patient.
| CAR T Cell Manufacturing Step | Duration |
|---|---|
| T Cell Collection | 3-6 hours |
| Genetic Modification | 7-10 days |
| CAR T Cell Multiplication | 10-14 days |
Step 4: Infusion of CAR T Cells
The last step is the car t cell infusion. Before infusion, patients get chemotherapy to reduce T cells. This makes room for the new CAR T cells. The cells are then infused into the patient’s bloodstream to fight cancer.
“The CAR T cell infusion was a turning point in my cancer journey. Knowing that my own immune cells were fighting the cancer gave me hope and strength.” – Sarah, CAR T cell therapy recipient
Throughout the therapy, patients are watched closely by healthcare teams. This is to manage side effects and ensure the best results.
Advantages of CAR T Cell Therapy Over Traditional Cancer Treatments
CAR T cell therapy is a new way to fight cancer. It uses the body’s immune system to target cancer cells. This makes it a more personalized and effective treatment.
Traditional treatments like chemotherapy and radiation harm both cancer and healthy cells. This can cause severe side effects and lower the patient’s quality of life. CAR T cell therapy, on the other hand, focuses only on cancer cells. This reduces damage to healthy tissues and organs.
Targeted Approach to Cancer Treatment
One big plus of CAR T cell therapy is its targeted approach. Genetically modified T cells are made to find and kill specific cancer cells. This precision helps CAR T cells target cancer cells without harming healthy cells.
The targeted nature of CAR T cell therapy brings several benefits:
- Less side effects compared to chemotherapy and radiation
- More effective in killing cancer cells
- Potential for long-term remission in patients with advanced blood cancers
Potential for Long-Term Remission
Another big advantage of CAR T cell therapy is its ability to lead to long-term remission. Clinical trials have shown impressive results. Some patients have achieved complete remission and stayed cancer-free for a long time.
The table below shows the long-term remission rates for different blood cancers treated with CAR T cell therapy:
| Blood Cancer Type | Complete Remission Rate | Median Duration of Response |
|---|---|---|
| Acute Lymphoblastic Leukemia (ALL) | 80-90% | 12-18 months |
| Diffuse Large B-Cell Lymphoma (DLBCL) | 40-50% | 8-12 months |
| Chronic Lymphocytic Leukemia (CLL) | 30-40% | 6-12 months |
These results are encouraging, but it’s important to remember that CAR T cell therapy is a new treatment. We need more long-term data to fully understand its benefits and risks. Ongoing research and clinical trials are working to improve its safety and effectiveness.
Potential Side Effects and Risks of CAR T Cell Therapy
CAR T cell therapy is a breakthrough in treating blood cancers. Yet, it’s important for patients and their families to know the possible side effects and risks. Common issues include cytokine release syndrome (CRS), neurological problems, and B cell aplasia. Understanding these risks helps in making informed decisions about this treatment.
Cytokine Release Syndrome (CRS)
Cytokine release syndrome is a common side effect of CAR T cell therapy. It happens when the immune system overreacts, releasing a lot of inflammatory molecules. Symptoms range from mild fever and flu-like symptoms to severe issues like low blood pressure and organ problems.
CRS is graded from 1 to 4, with higher grades meaning more severe symptoms. Doctors closely watch for signs of CRS and offer supportive care. This may include:
- Medications to reduce fever and inflammation
- Oxygen support or mechanical ventilation for respiratory distress
- Vasopressors to maintain blood pressure
- Tocilizumab, an antibody that blocks the effects of IL-6, a key cytokine involved in CRS
Neurological Toxicities
Neurological toxicities, or ICANS, can happen in some patients. Symptoms include confusion, tremors, seizures, or even coma. The exact cause is not fully understood, but it’s thought to be related to CAR T cells in the brain and cytokines affecting neural function.
Managing these toxicities involves monitoring the patient’s neurological status closely. Supportive care may include:
- Anti-seizure medications
- Corticosteroids to reduce inflammation
- Intensive care support for severe cases
B Cell Aplasia
B cell aplasia is a side effect of CAR T cell therapy targeting CD19 antigen. It affects both cancerous and healthy B cells. This leads to a decrease in B cells, which are important for fighting infections.
To manage B cell aplasia, patients get regular IVIG infusions. This provides them with antibodies to protect against infections. The duration of B cell aplasia varies, but it can last several years.
| Side Effect | Symptoms | Management Strategies |
|---|---|---|
| Cytokine Release Syndrome (CRS) | Fever, low blood pressure, difficulty breathing, organ dysfunction | Medications to reduce fever and inflammation, oxygen support, vasopressors, tocilizumab |
| Neurological Toxicities (ICANS) | Confusion, tremors, seizures, coma | Anti-seizure medications, corticosteroids, intensive care support |
| B Cell Aplasia | Depletion of healthy B cells, increased risk of infections | Regular intravenous immunoglobulin (IVIG) infusions |
While CAR T cell therapy has significant side effects and risks, healthcare providers are skilled in managing them. By monitoring patients closely and providing the right care, these risks can be minimized. This allows patients to benefit from this potentially life-saving treatment. As research continues, strategies for managing CAR T cell therapy risks will improve, making it safer and more accessible.
Current FDA-Approved CAR T Cell Therapies
CAR T cell therapy has changed how we treat some blood cancers. The U.S. Food and Drug Administration (FDA) has approved two CAR T cell therapies: Kymriah and Yescarta. These treatments have shown great results in patients with certain blood cancers.
Kymriah (tisagenlecleucel) was the first to get FDA approval in August 2017. It’s for kids and young adults with B-cell acute lymphoblastic leukemia (ALL) that didn’t respond to other treatments. In May 2018, it also got approval for adults with a type of lymphoma.
Yescarta (axicabtagene ciloleucel) got FDA approval in October 2017. It’s for adults with certain types of lymphoma, including DLBCL and primary mediastinal B-cell lymphoma.
“The approval of Kymriah and Yescarta marks a significant milestone in the field of cancer immunotherapy and offers new hope for patients who have exhausted conventional treatment options.”
Studies have shown Kymriah and Yescarta work well. In the ELIANA trial, Kymriah helped 81% of young patients with B-cell ALL. The ZUMA-1 trial showed Yescarta worked for 82% of patients with refractory large B-cell lymphoma.
The success of Kymriah and Yescarta has opened doors for more research. Many trials are looking into using CAR T cell therapy for other cancers. As more research comes in, CAR T cell therapy is expected to change how we treat blood cancers.
Ongoing Clinical Trials and Future Developments in CAR T Cell Therapy
CAR T cell therapy is showing great promise in treating blood cancers. Researchers are now looking to use it for more cancers and to make it safer and more effective. Many clinical trials are underway to explore these possibilities.

Expanding to Other Types of Cancer
So far, CAR T cell therapy has mainly been used for blood cancers like leukemia and lymphoma. But, scientists are now testing it on solid tumors. They are looking at using CAR T cells to target specific proteins on solid tumors, such as:
- HER2 for breast cancer
- EGFR for lung cancer
- PSMA for prostate cancer
- GD2 for neuroblastoma
Improving Safety and Efficacy
Researchers are also working on making CAR T cell therapy safer and more effective. They are exploring ways to reduce side effects and improve how well it works. Some ideas include:
- Creating “off-switch” mechanisms to control CAR T cell activity
- Adding suicide genes to eliminate CAR T cells if needed
- Modifying CAR designs to be more specific and reduce side effects
- Looking into using different cell types, like NK cells or allogeneic T cells
Combination Therapies with CAR T Cells
Combining CAR T cell therapy with other treatments might make it even better. Scientists are studying how CAR T cells work with:
| Combination Therapy | Potential Benefits |
|---|---|
| Checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4) | Help CAR T cells last longer and work better |
| Targeted therapies (e.g., kinase inhibitors) | Make tumors more visible to CAR T cells |
| Chemotherapy or radiation therapy | Shrink tumors and help CAR T cells do their job |
As we look to the future, car t cell therapy clinical trials will keep shaping this new treatment. With ongoing research, we can expect more breakthroughs soon. This brings hope to those fighting various cancers.
What is the CAR T Cell Therapy: A Comprehensive Overview
CAR T cell therapy is a new way to fight cancer. It uses a patient’s own immune system to attack cancer cells. This method changes T cells to target and kill cancer cells, mainly in blood cancers like leukemia and lymphoma.
To start, T cells are taken from the patient. Then, they are changed in a lab to find and kill cancer cells. This makes millions of cells ready to fight cancer.
These CAR T cells are given back to the patient. They find and destroy cancer cells. This is more precise than old treatments like chemotherapy, which can harm healthy cells too.
The journey of CAR T cell therapy has been exciting. Here are some key moments:
| Year | Milestone |
|---|---|
| 1989 | First CAR T cell concept proposed |
| 2010 | First clinical trial of CAR T cells in humans |
| 2017 | FDA approves first CAR T cell therapy (Kymriah) |
| 2020 | Over 500 clinical trials involving CAR T cells ongoing worldwide |
While CAR T cell therapy is a big success for some blood cancers, it has risks. Side effects include CRS, neurological problems, and B cell aplasia. But, scientists are working hard to make it safer and more effective for more cancers.
CAR T cell therapy is a big step forward in cancer treatment. It gives hope to those who have tried everything else.
The future of CAR T cell therapy is bright. It could change how we treat cancer and help more patients. This guide covers the basics, history, and what’s next in this exciting field.
Eligibility Criteria for CAR T Cell Therapy
CAR T cell therapy is a breakthrough in treating some blood cancers. But, not everyone can get this treatment. Doctors carefully check many factors to see if a patient is a good fit for CAR T cell therapy.
Patient Selection Process
The criteria for CAR T cell therapy include:
- Diagnosis of specific blood cancers like DLBCL, primary mediastinal B-cell lymphoma, or ALL
- Having tried all standard treatments or failed after previous therapies
- Being 18 or older for most CAR T cell products
- Having good organ function, like the heart, lungs, kidneys, and liver
- No active infections or serious health issues that could make treatment hard
A team of doctors, including oncologists and hematologists, review each case. They decide if a patient is eligible for CAR T cell therapy.
Pre-Treatment Evaluations
Before starting CAR T cell therapy, patients go through many tests. These tests check their health and if they’re ready for the treatment. The tests might include:
- Complete blood count and chemistry panel to check organ function
- Bone marrow biopsy to see how much cancer is there and get cells for CAR T cell making
- Imaging tests, like PET/CT scans, to see how big the cancer is
- Cardiac function tests, such as echocardiogram or MUGA scan, to check the heart
- Pulmonary function tests to see how well the lungs work
- Infectious disease screening to make sure there are no active infections
| Pre-Treatment Evaluation | Purpose |
|---|---|
| Complete blood count and chemistry panel | Check organ function |
| Bone marrow biopsy | See how much cancer is there and get cells for CAR T cell making |
| Imaging tests (PET/CT scans) | Find out how big the cancer is |
| Cardiac function tests | Check the heart’s function |
| Pulmonary function tests | See how well the lungs work |
| Infectious disease screening | Make sure there are no active infections |
These tests help doctors see if a patient is a good candidate for CAR T cell therapy. They also look for any risks or problems that might happen during treatment. By choosing the right patients, doctors can make sure the treatment works well and is safe.
Patient Experience and Quality of Life After CAR T Cell Therapy
Patients often wonder about the recovery and life changes after CAR T cell therapy. Many report a big improvement in their quality of life after treatment.
Right after the CAR T cell infusion, patients might feel side effects like cytokine release syndrome (CRS) or neurological issues. But, the healthcare team manages these side effects, and they usually go away in a few weeks. As patients get better, they start to feel more energetic and overall better.
One big plus of CAR T cell therapy is the chance for long-term remission. Patients who get better often feel hopeful and can do things they loved before cancer. Here’s what one patient said:
After my CAR T cell therapy, I felt like I had a second chance at life. I was able to go back to work, spend time with my family, and even take a vacation – things I never thought I’d be able to do again.
Recovery is different for everyone, but many see their quality of life improve after CAR T cell therapy. The table below shows some of these improvements:
| Quality of Life Metric | Before CAR T Cell Therapy | After CAR T Cell Therapy |
|---|---|---|
| Energy levels | Low | Improved |
| Physical functioning | Limited | Enhanced |
| Emotional well-being | Stressed, anxious | Hopeful, optimistic |
| Social engagement | Reduced | Increased |
It’s key for patients to stay close to their healthcare team during recovery. Going to all follow-up appointments helps ensure the best outcomes and quality of life after CAR T cell therapy.
Cost and Insurance Coverage for CAR T Cell Therapy
The cost of CAR T cell therapy is a big worry for those thinking about it. This treatment is very personalized and complex, making it pricey. It’s important for patients and their families to know about the costs and insurance coverage.
CAR T cell therapies are among the most expensive cancer treatments today. The prices for FDA-approved CAR T cell products range from $373,000 to $475,000 per treatment. These prices reflect the complex process, the personalized nature, and the high research and development costs.
Current Pricing of CAR T Cell Treatments
| CAR T Cell Product | Manufacturer | List Price |
|---|---|---|
| Kymriah | Novartis | $475,000 |
| Yescarta | Kite Pharma (Gilead) | $373,000 |
| Tecartus | Kite Pharma (Gilead) | $373,000 |
| Breyanzi | Bristol Myers Squibb | $410,300 |

Many insurance companies, including Medicare and Medicaid, cover FDA-approved CAR T cell therapies. Private insurers are also starting to cover these treatments. They see the value in helping patients with certain blood cancers.
Insurance Coverage and Reimbursement Policies
Insurance coverage for CAR T cell therapy depends on the plan and the patient’s needs. Coverage is decided on a case-by-case basis. Factors include the type of cancer, the disease stage, previous treatments, and the patient’s overall health.
- Type of cancer
- Stage of the disease
- Previous treatments
- Overall health of the patient
Patients should talk to their healthcare providers and insurance companies to understand their options and any costs they might face.
As CAR T cell therapies show their worth in treating blood cancers, more insurance coverage is expected. This will help make the treatment available to more patients in need.
The Role of Healthcare Providers in CAR T Cell Therapy
CAR T cell therapy is changing how we fight cancer. Healthcare providers are key to making it work. They help from the start to aftercare, working together for the best results.
Because CAR T cell therapy is complex, many specialists are needed. This includes oncologists, hematologists, nurses, and more. A study in the Journal of Clinical Medicine shows how important advanced practice providers are. They help manage the care needed by these patients.
Multidisciplinary Team Approach
Good care for CAR T cell patients needs teamwork. Each team member has a special role. Here are some of them:
- Oncologists and hematologists plan the treatment
- Apheresis specialists collect T cells
- Cell processing technicians make the T cells ready
- Nurses give the treatment and watch for side effects
- Social workers and psychologists offer emotional support
Patient Education and Support
Healthcare providers teach patients and families about CAR T cell therapy. They explain what to expect and offer support. It’s important to talk clearly about the treatment, its risks and benefits, and what comes next.
“Our goal is to provide complete, personal support for each patient getting CAR T cell therapy. By working together, we ensure patients get the best care and outcomes.”
As CAR T cell therapy gets better, providers must keep learning. They need to know the latest research and best practices. This way, they can help patients get the most from this new cancer treatment.
Comparing CAR T Cell Therapy to Other Immunotherapy Approaches
When looking at cancer treatment options, it’s key to know how CAR T cell therapy stacks up against others. All immunotherapies use the immune system to fight cancer. But, they work in different ways and target different cancers.
Checkpoint inhibitors and adoptive cell transfer (ACT) therapies are often compared to CAR T cell therapy. Let’s dive into each:
Checkpoint Inhibitors
Checkpoint inhibitors block proteins that stop immune cells from attacking cancer. These proteins, or checkpoints, slow down the immune system. By stopping these checkpoints, the immune system can better attack and destroy cancer cells. Examples include:
- PD-1 inhibitors (e.g., pembrolizumab, nivolumab)
- PD-L1 inhibitors (e.g., atezolizumab, durvalumab)
- CTLA-4 inhibitors (e.g., ipilimumab)
These treatments have been very successful in treating solid tumors like melanoma and lung cancer. But, they might not work as well for blood cancers, where CAR T cell therapy has shown great promise.
Adoptive Cell Transfer (ACT) Therapies
ACT therapies collect immune cells from a patient, modify them in the lab, and then give them back to fight cancer. CAR T cell therapy is a form of ACT that uses genetically modified T cells. Other types include:
- Tumor-infiltrating lymphocyte (TIL) therapy
- T cell receptor (TCR) engineered T cell therapy
- Natural killer (NK) cell therapy
These therapies are similar to CAR T cell therapy in their process. But, they differ in the immune cells used and how they’re modified to target cancer.
| Immunotherapy | Mechanism of Action | Cancers Treated |
|---|---|---|
| CAR T Cell Therapy | Genetically modified T cells target specific antigens on cancer cells | Blood cancers (leukemia, lymphoma, multiple myeloma) |
| Checkpoint Inhibitors | Block proteins that inhibit immune response against cancer cells | Solid tumors (melanoma, lung cancer, renal cell carcinoma) |
| Other ACT Therapies | Collect, modify, and reinfuse immune cells to fight cancer | Various solid tumors and blood cancers, depending on the specific therapy |
As research continues, there’s growing interest in mixing CAR T cell therapy with other immunotherapies. This could make treatments more effective and treat more types of cancer. Knowing the strengths and weaknesses of each is key to creating personalized treatment plans that work best for patients.
Real-World Patient Stories and Testimonials
CAR T cell therapy has changed lives for many battling blood cancers. Emily Whitehead was just five when she got acute lymphoblastic leukemia. Her family tried everything, then turned to CAR T cell therapy. It worked, and Emily has been cancer-free for over 10 years.
Doug Olson’s story is also inspiring. He was diagnosed with chronic lymphocytic leukemia in 1996. In 2010, he joined a CAR T cell therapy trial. Now, he’s cancer-free, showing the therapy’s long-term benefits.
These stories highlight the human side of cancer treatment. They give hope to those fighting cancer and push researchers to keep improving CAR T cell therapy. As more people share their experiences, understanding and support for this treatment will grow.
FAQ
Q: What is CAR T cell therapy?
A: CAR T cell therapy is a new way to fight cancer. It uses the body’s immune system to attack cancer cells. This is done by changing T cells to find and kill cancer cells.
Q: How does CAR T cell therapy work?
A: First, T cells from the patient are taken. Then, they are changed to find cancer cells. These cells are grown in a lab and put back into the patient. They then go after and kill cancer cells.
Q: What types of cancer can be treated with CAR T cell therapy?
A: CAR T cell therapy is mainly for blood cancers like leukemia and lymphoma. But, research is going on to use it for other cancers too.
Q: What are the advantages of CAR T cell therapy over traditional cancer treatments?
A: CAR T cell therapy targets cancer cells more precisely. It also might lead to long-term remission for some patients. This is good news for those who have tried other treatments without success.
Q: What are the possible side effects and risks of CAR T cell therapy?
A: Side effects can include cytokine release syndrome (CRS), brain problems, and low B cell count. But, these can be managed with good care and monitoring.
Q: Who is eligible for CAR T cell therapy?
A: Who can get CAR T cell therapy depends on their cancer type, how far it has spread, and their health. Doctors carefully check each patient to see if they’re a good fit.
Q: How much does CAR T cell therapy cost, and is it covered by insurance?
A: CAR T cell therapy is very expensive, costing hundreds of thousands to millions of dollars. Insurance policies vary, so patients should talk to their doctors and insurance about costs.
Q: What is the role of healthcare providers in CAR T cell therapy?
A: Healthcare providers are key in CAR T cell therapy. They work together to make sure patients get the best care. They handle everything from choosing patients to educating and supporting them.
Q: Are there any real-world patient stories or testimonials about CAR T cell therapy?
A: Yes, many patients have had amazing results from CAR T cell therapy. Their stories show the treatment’s power and offer hope to others fighting cancer.